Abstract
Introduction: Hemophagocytic lymphohistiocytosis (HLH) is a rare and life-threatening hyperinflammatory syndrome induced by aberrantly activated myeloid and T cells. HLH may be primary (i.e., due to genetic mutations) which typically presents in children, or secondary (i.e., triggered by an infection, malignancy, or rheumatologic condition) which is most common in adults. Without treatment, HLH is universally fatal; even with existing treatment, overall mortality ranges from 50-75%. Most of our understanding of HLH comes from studies in the pediatric population, and limited information is available on the diagnosis and treatment of patients with secondary HLH (sHLH). The aim of this study is to compile a historical cohort of sHLH patients from large US medical systems to better understand and refine the sHLH clinical definition, describe the epidemiology (including incidence, prevalence, and natural history of the disease), and characterize demographic, clinical, treatment characteristics, survival, and healthcare resource utilization (HRU) of the cohort compared with a matched comparison group.
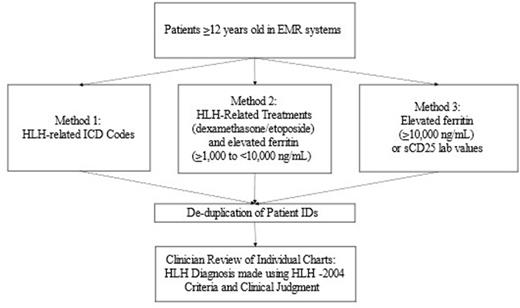
Methods: A historical cohort study was designed to identify sHLH patients aged ≥12 years from de-identified Electronic Medical Record (EMR) systems at health care centers in the US. The EMR systems track patients longitudinally over multiple years and are linked at the patient level by a unique identifier that is consistent across service locations and time. Three different methods (Figure 1) and chart reviews were used to enable identification of all possible sHLH patients and to better understand initial recorded diagnosis, differences in treatment and outcomes, and to determine a simpler system for accurate identification of sHLH cases in the healthcare system. As sHLH is a rare disorder that can be complex to diagnose and treat, these algorithms were developed to identify patients that may or may not have been assigned an ICD code for HLH. Approximately 5.4 million unique patient records were reviewed. Comparison of patients without sHLH were also identified from the EMR systems, matched on birth year, sex, and county of residence.
Results: Patient electronic data from 2009-2021 were reviewed using all three methods. Of >800,000 unique inpatient records, 903 unique de-duplicated patients were identified for chart review. 57 patients were identified using HLH-related ICD codes (Method 1). An additional 846 patients were identified using HLH-related treatments (dexamethasone and etoposide) and elevated ferritin (≥1,000 to <10,000 ng/mL) in patient charts (Method 2) and/or by elevated ferritin (≥10,000 ng/mL) or sCD25 lab test ordered (with cohorts stratified by sCD25 lab values ≤3,900 U/mL and >3,900 U/mL) (Method 3). Potential HLH patient charts were then adjudicated via clinician review using HLH-2004 criteria and clinical judgment.
Conclusion: Since sHLH is a rare, heterogenous, and complex disease to diagnosis and treat, HLH-related ICD codes (Method 1) may not be sufficient to capture the spectrum of sHLH patients. Both HLH-related treatments in charts (Method 2) and elevated ferritin and sCD25 values (Method 3) could prove useful in identifying additional sHLH patients. Moreover, Method 3 could show promise as a simplified strategy for identifying and diagnosing patients with sHLH. This process could inform the clinical definition and true burden of sHLH, particularly those patients who never received an sHLH diagnosis, as well as the clinical, demographic, and treatment characteristics and outcomes of this unique patient population.
Disclosures
Broome:Electra Therapeutics: Consultancy; Alexion Pharmaceuticals: Honoraria; argenx: Honoraria; Annexon Biosciences: Honoraria; Incyte Corporation: Honoraria; Sanofi: Honoraria. Fryzek:Electra Therapeutics: Research Funding. Bylsma:Electra Therapeutics: Research Funding. Mitchell:Electra Therapeutics: Research Funding. Shara:Electra Therapeutics: Research Funding. Fernandez:Electra Therapeutics: Research Funding. Catlett:Electra Therapeutics: Research Funding. Edwards:Electra Therapeutics: Research Funding. Hausrath:Electra Therapeutics: Research Funding. Lipworth:Electra Therapeutics: Research Funding. Lai:Electra Therapeutics: Current Employment. Dong:Electra Therapeutics: Current Employment. Daver:Agios, Celgene, SOBI and STAR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos and Jazz Pharmaceuticals: Other: Data monitoring committee member; Karyopham Therapeutics and Newave Pharmaceutical: Research Funding; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Novartis, Jazz, Amgen, Servier, Karyopharm, Trovagene, Trillium, Syndax, Gilead, Pfizer, Bristol Myers Squibb, Kite, Actinium, Arog, Immunogen, Arcellx, and Shattuck: Consultancy, Other: Advisory Role; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Gilead, Immunogen, Pfizer, Bristol Myers Squibb, Trovagene, Servier, Novimmune, Incyte, Hanmi, Fate, Amgen, Kite, Novartis, Astex, KAHR, Shattuck, Sobi, Glycomimetics, Trillium: Research Funding. Allen:Electra Therapeutics: Consultancy; Sobi, Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal